Crystals, Jewellry and Chemistry
Nearly all of us have jewellery of some kind, whether it be a wedding or engagement ring, a nice watch, a pendant or locket or even body jewellery. However what a lot of people don't think about is the materials that go into these pieces. I'm fascinated by jewellery and gemstones, as I see it as one of those wonderful overlaps between science and art. Knowledge of metallurgy, crystallography, deft hands and an eye for what looks attractive are all required to create pieces that people will want to buy. This is especially important when the customer will be wearing the piece for the rest of their live, you need it not to fall apart and to stay looking fashionable and pretty. In this post I'll go over some metals used in jewellery, some of my favourite gemstones and some jewellers terms which you may have heard, but may not know what they mean.

Metallurgy is the branch of science concerned with the properties and purification of the metallic elements. As you can imagine this is hugely important for jewellers, as they want the metal that they use to be durable, attractive and workable. Fortunately, the group 11 elements tick these boxes (mostly, as we shall see).
Silver (Ag), atomic number 47:

Silver has been known since antiquity (one of the seven "metals of antiquity" alongside gold, copper, tin, lead, iron and mercury). Due to its bright colour and relative scarcity it is considered a precious metal and has held cultural significance since ancient times. Pure silver is quite soft (Mohs Hardness = 2.5) and can be scratched with your fingernail. Obviously this isn't useful for say a ring which will get regularly knocked around.
To overcome this, silver in jewellery is often what is called "sterling silver". This means that it is at least 92.5 % silver, alloyed with copper. It is denoted on pieces with the mark "925". This alloying is a commonly used practice for jewellers to make pieces more hard wearing, indeed it is also done with the next metal, gold.
Gold (Au), atomic number 79.
Similarly to silver, gold is an ancient metal and has been regarded as precious. It is sought after due to its beautiful yellow colour and, due to its high density, it is perceived to be valuable. Consider holding a 50p coin, it weighs 8 g. A 50p coin made of pure gold would weigh nearly three times as much at 22 g.
Gold is also quite soft however, and like silver is almost never used in its pure form in jewellery. Gold purity is often rated using the karat system, you may have heard someone described as a "24 karat guy" to mean that they are a good (golden) person. This is not to be confused with a carat (annoying terminology, I know) which is a measure of mass of gemstones.
24k gold is pure gold (actually from 99.95 % can be called 24k), the next common denomination is 18k gold, which means it is 18/24 parts gold (75 %) and 6 parts something else (for example copper or silver). This goes down to 9k which is half as much gold.
Now we have our metal, what about our stone?
Emerald is my favourite gemstone, I like it because of its colour and the fact that a high quality emerald is a rare thing to see. Before we see why, let me just tell you a little bit about crystals.
Crystals are solids in which the constituents are arranged in a highly-ordered, microscopic arrangement which extends in all directions. If all crystals were perfect, that is to say that there were no gaps or inclusions in these lattices, the world would be a very different place. For example, most gemstones would not exist as they do now.
Emerald is a type of beryl, the mineral with chemical formula Be3Al2(SiO3)6. It is naturally colourless, and is only called emerald when there are chromium atoms within the lattice which give it its green colour. Indeed if there were Mn2+ ions present instead, the colour would be pink and the gem would be called morganite.
Due to the structure of emerald, with many fissures, they are often "included". This means that they are not transparent due to impurities within the stone, hence a clear emerald is rare.
If green isn't your colour, then we can always go for a classic diamond. Diamond is the hardest known material, meaning it will scratch any other material, and is therefore used in cutting tools. It is also a very simple stone, with chemical formula of C. This means that the repeating unit of diamond is simply a carbon atom. The atoms are arranged tetrahedrally (see figure below) and are bound by covalent bonds.
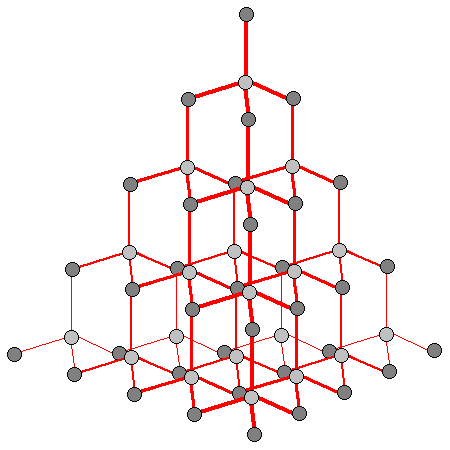
However its interesting structure and formula aren't why it is used in jewellery, diamond has a high optical dispersion, meaning it can split white light into its constituent parts. This is what gives diamond its sparkle, and why it is a coveted material!
If you’re interested in hearing about my work, you can follow me on Twitter @StubbingScience and the work of GlamSci can be found @GlamSci.



